Can you solve it?
Microtubule associated-protein tau (tau for short) is a microtubule stabilizing protein most commonly found in neurons. Tau helps regulate transport of other proteins by binding or falling off of microtubules.
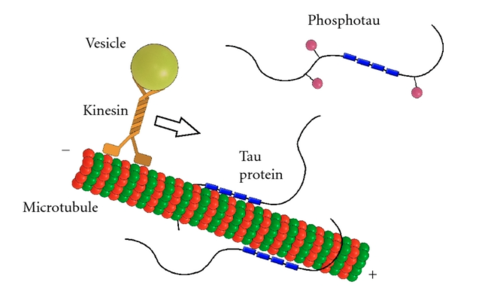
The binding affinity and cellular localization of tau can vary, and is regulated by many post-translational modifications (PTMs), including ubiquitination, SUMOylation, acetylation, and phosphorylation. These PTMs act like little switches on a microchip, and are attached and removed by enzymes like kinases, phosphatases, acetyltransferases, deacetylases and ubiquitin ligases that are in turn regulated by external stimuli including insulin signaling, glutamate, GABA, and neuropeptide receptors.
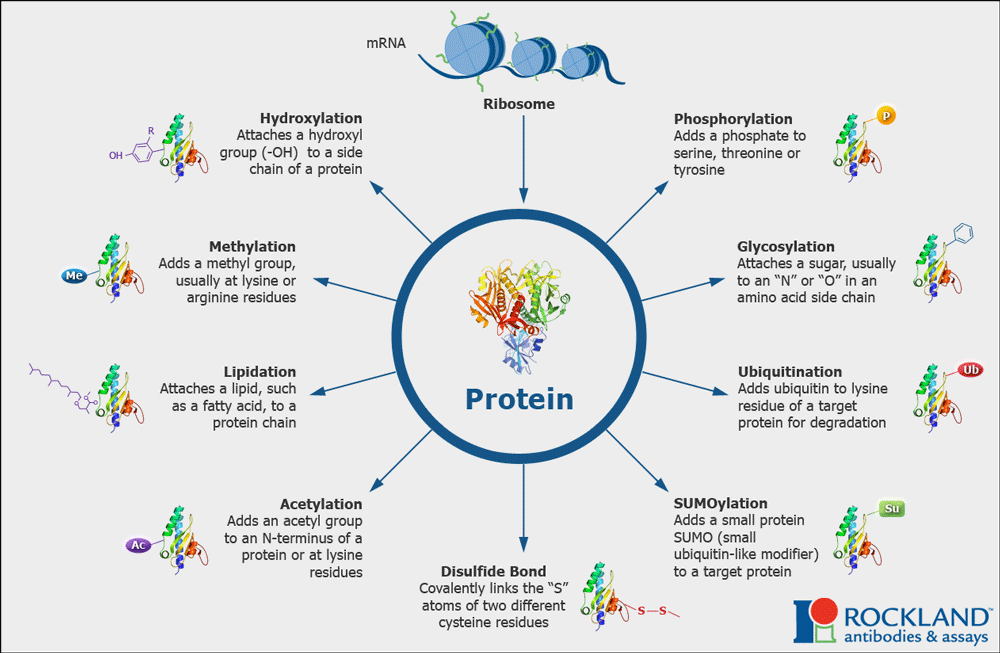
Mixing and matching of PTMs dynamically regulates neuronal polarization and dendritic branching. When it goes awry, the cell makes too much aggregate-prone, hyperphosphorylated species of tau, a common hallmark of Alzheimer’s disease. Hyperphosphorylated tau clumps with more tau to form tangles, which cause cell death and increase inflammation in the brain. All the different PTMs and their relative effects on tau make for an incredibly complex profile, which we have taken to calling the “PTM code”.
Crack the Code!
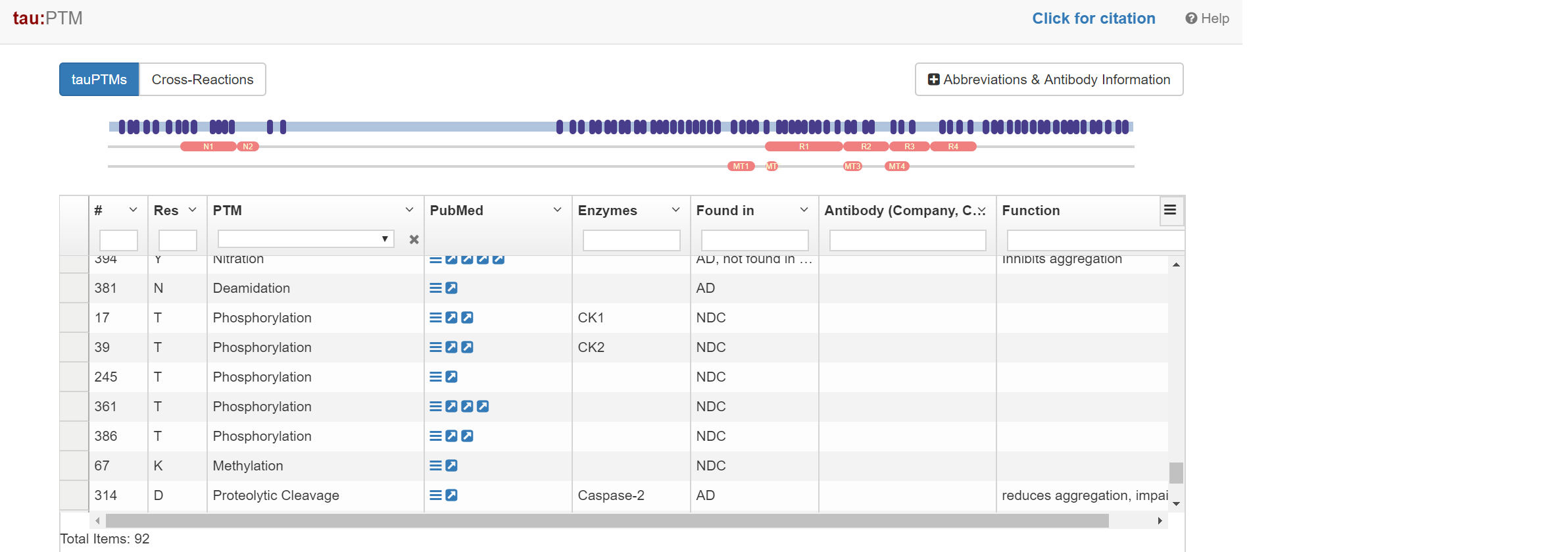
Someone at BioMed took the time to build a huge database of tau PTMs. Each one is mapped to the amino acid sequence of tau, and has the citation linked. Check it out to see if you can solve the tau post translational modifcation puzzle, and crack the tau code!