Our Research Mission in Todd Cohen’s Lab at UNC Chapel Hill
One-liner: To identify the pathogenic mechanisms underlying neurodegenerative disease.
Although distinct, many of these diseases share common underlying pathogenic mechanisms. We seek to uncover the molecular pathways that promote protein aggregation and the formation of amyloid deposits that cause neurodegeneration and cognitive impairments.
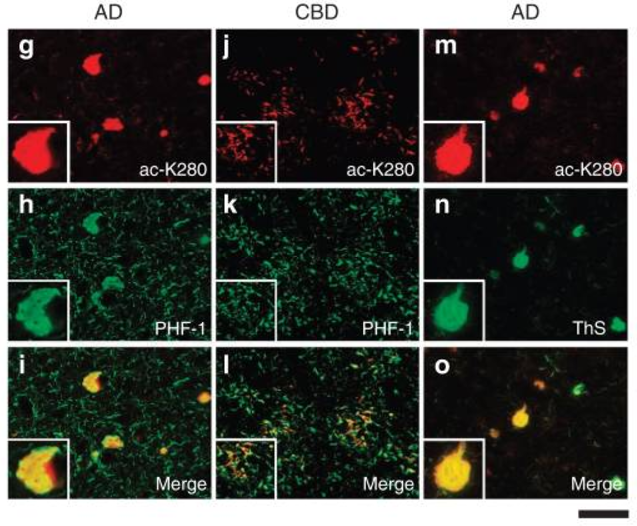
Using a combination of biochemistry, genetics, and cell biology, we have identified several post-translational mechanisms including lysine acetylation, phosphorylation, and cysteine disulfide modifications that critically regulate many disease-associated proteins in the brain including the tau protein in AD and the TDP-43 protein present in ALS and FTLD patients. We have pioneered the concept that lysine acetylation, in particular, is an unanticipated, yet critical modification that promotes the evolution and maturation of pathological aggregates in a spectrum of neurodegenerative diseases.
This raises the intriguing possibility of modification-targeted therapeutics to combat normal ageing mechanisms and a broad range of neurodegenerative diseases. Ultimately, by uncovering the mechanistic details that regulate normal and aberrant protein functions in the diseased brain, can we begin to uncover the molecular platform for future drug-based therapies against these debilitating diseases.
Relevance:
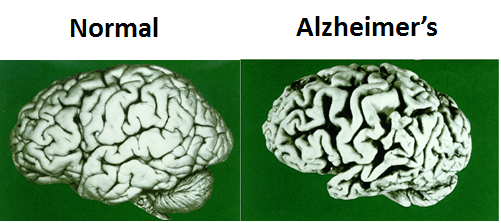
Although clinically and pathologically distinct, the disorders shown below share a common underlying pathogenic mechanism in which normally soluble proteins become abnormally sequestered into protein aggregates that can exert toxic loss and gain of functions in a tissue-specific manner.
Current research projects:
1) Tau-mediated neurodegeneration in Alzheimer’s disease
2) TDP-43 mediated neurodegeneration in ALS
TDP-43 modifications similarly promote aberrant pathology seen in diseased brain and spinal cord of ALS and FTLD-TDP patients. Understanding such post-translational mechanisms could lead to better therapeutic avenues to pursue for ALS, FTD and other diseases characterized by TDP-43 pathology.
3) Altered post-translationally modified proteome (PT-MAP) in neurodegenerative diseases.
Please contact me if you’re interested in contributing to our research mission!
Keep up to date on our science here.
-

Kyle Pellegrino
Kyle is an MD/PhD student who completed the first two years of medical school at UNC and joined the Cohen Lab through the Department of Pharmacology in 2022. As an undergraduate, he attended Cornell University, where he … Read more
-

Nicholas Zullo
Nicholas Zullo (he/him/his) is a recent graduate of UNC-Chapel Hill and current master’s student at Duke University. He is very interested in neurodegenerative research, particularly ALS, and plans to either pursue research or attend medical school in the future. … Read more
-

Darien Campisi
Darien is a graduate student at NC State University, planning to graduate with her Master’s of Physiology in December 2022. She graduated from UNC Chapel Hill with a B.S. in Biology in May 2021 and has been in the … Read more
-

Dr. Cohen to present at First “SLAM-DUNC” Symposium
On June 25th, 2022 Dr. Cohen will be presenting as a keynote speaker as part of the first Symposium for Learning about Alzheimer’s disease-related Medical research at Duke and UNC (SLAM-DUNC), hosted by the Duke-UNC Alzheimer’s Disease Research Center. It … Read more