The Scientist’s Rising Stars: Dr. Todd Cohen!
Dr. Cohen was recognized as a Rising Star from The Scientist!
In the November 2019 issue of The Scientist on pages 16 – 17, Full PDF Here
Dr. Cohen was recognized as a Rising Star from The Scientist!
In the November 2019 issue of The Scientist on pages 16 – 17, Full PDF Here
 Dr. Cohen has been in the news a lot lately! Here’s him on another radio show, called Radio in Vivo! A great way to learn about Dr. Cohen’s inspiration and drive to enter Alzheimer’s disease research. Click the picture or here to check it out.
Dr. Cohen has been in the news a lot lately! Here’s him on another radio show, called Radio in Vivo! A great way to learn about Dr. Cohen’s inspiration and drive to enter Alzheimer’s disease research. Click the picture or here to check it out.
Dr. Todd Cohen was featured in “The News and Notes Podcast” by Jeffrey C. McAndrew, where they discussed new, promising discoveries in ALS and what a typical day is like in the Cohen Lab at UNC Chapel Hill.
Press play to listen in on the conversation!
Or click here for the full podcast webpage
Dr. Todd Cohen and the efforts of the Cohen Lab were mentioned in the Oshkosh Herald! The story below features the efforts of Gary Beyer, a patient with sporadic Inclusion Body Myositis (sIBM), a muscle weakening disease that is similar in molecular origin to Amyotropic Lateral Sclerosis (ALS).
The article also mentions a Podcast Conversation with Dr. Todd Cohen on similar subjects
To read the full story, click the pdf link below!

Click here to download- Oshkosh Newsletter page 3
Congratulations to Candice Crilly and Hanna Trzeciakiewicz for receiving Predoctoral Fellowships from the National Science Foundation!
The National Science Foundation predoctoral fellowships are designed to help ensure the vitality of the human resource base of science and engineering in the United States and reinforces its diversity. The program recognizes and supports outstanding graduate students in NSF-supported science, technology, engineering, and mathematics disciplines who are pursuing research-based master’s and doctoral degrees at accredited US institutions.
Microtubule associated-protein tau (tau for short) is a microtubule stabilizing protein most commonly found in neurons. Tau helps regulate transport of other proteins by binding or falling off of microtubules.
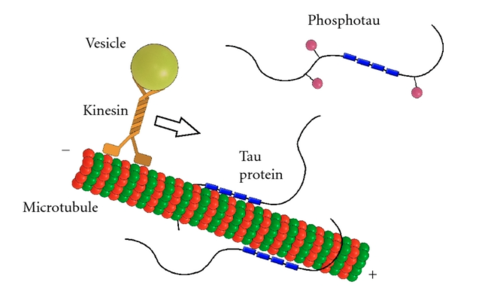
The binding affinity and cellular localization of tau can vary, and is regulated by many post-translational modifications (PTMs), including ubiquitination, SUMOylation, acetylation, and phosphorylation. These PTMs act like little switches on a microchip, and are attached and removed by enzymes like kinases, phosphatases, acetyltransferases, deacetylases and ubiquitin ligases that are in turn regulated by external stimuli including insulin signaling, glutamate, GABA, and neuropeptide receptors.
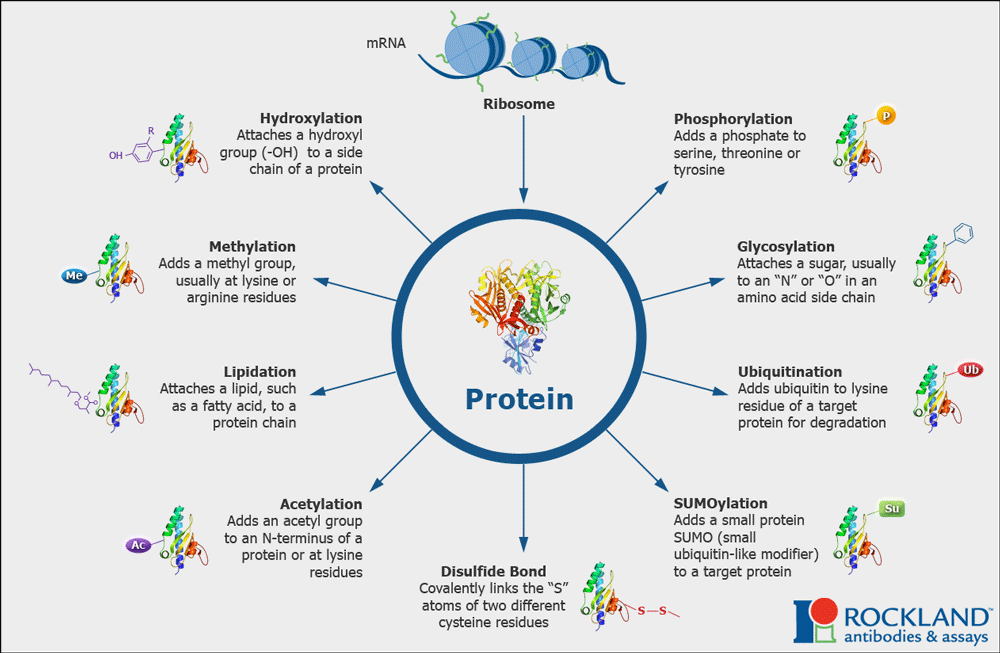
Mixing and matching of PTMs dynamically regulates neuronal polarization and dendritic branching. When it goes awry, the cell makes too much aggregate-prone, hyperphosphorylated species of tau, a common hallmark of Alzheimer’s disease. Hyperphosphorylated tau clumps with more tau to form tangles, which cause cell death and increase inflammation in the brain. All the different PTMs and their relative effects on tau make for an incredibly complex profile, which we have taken to calling the “PTM code”.
Someone at BioMed took the time to build a huge database of tau PTMs. Each one is mapped to the amino acid sequence of tau, and has the citation linked. Check it out to see if you can solve the tau post translational modifcation puzzle, and crack the tau code!
Check us out on YouTube!
The Cohen Lab just published a new article, The Deacetylase HDAC6 Mediates Endogenous Neuritic Tau Pathology in Cell Press, and the UNC School of Medicine Newsroom Interviewed Dr. Cohen on the article! A sneak peak:
Led by Todd Cohen, PhD, assistant professor of neurology, UNC scientists used human cell cultures to show how amyloid beta can trigger a dramatic inflammatory response in immune cells and how that interaction damages neurons. Then they showed how that kind of neuron damage leads to the formation of bead-like structures filled with abnormal tau protein. Similar bead-like structures are known to form in the brain cells of people with Alzheimer’s disease.
For the full UNC newsroom article, click here.
We were also featured in Medical News Today!
You can find their take on our work here. (Image source: Medical News Today)

For a full text PDF, click here.
Congrats to all the authors, especially the director of the project, Dr. Henry Tseng!
Enjoy!
Congrats to all working on the project! We are closer to understanding the molecular pathology at work in ALS!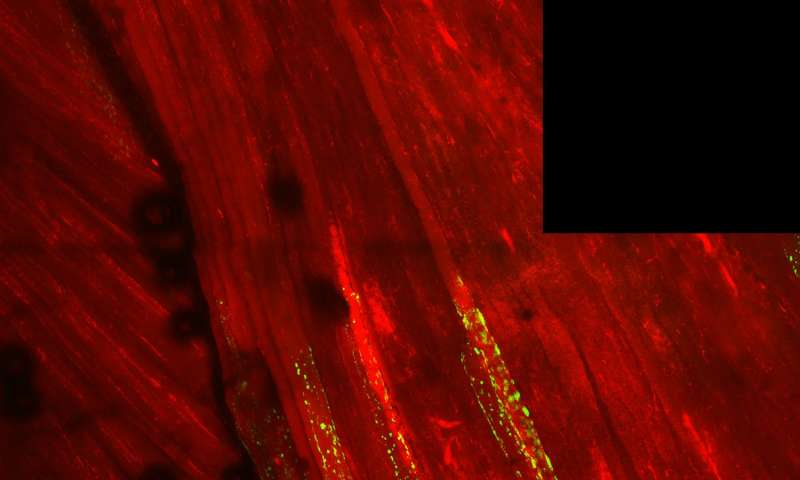
CHAPEL HILL, NC – Scientists have long known that a protein called TDP-43 clumps together in brain cells of people with amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s Disease, and is associated with neuron death. This same protein is thought to cause muscle degeneration in patients with sporadic inclusion body myositis (sIBM), leading many researchers to think that TDP-43 is one of the causative factors in ALS and sIBM. Now, UNC School of Medicine and NC State researchers found that a specific chemical modification called acetylation promotes TDP-43 clumping in animals. Using a natural anti-clumping method in mouse models, the scientists reversed protein clumping in muscle cells and prevented the sIBM-related muscle weakness.
Source: ALS: New Clues to the Cause and How Future Drugs Might Reverse Disease — News Room – UNC Health Care
#UNC and @NCState researchers team up & find new clues about the causes of ALS and how to reverse the disease: https://t.co/snFOYply1T pic.twitter.com/HezsbyqWyp
— UNC-Chapel Hill (@UNC) July 21, 2017
